Energy Properties of Hydrogen
This blog is based on the post Energy Properties of Hydrogen.
This is the third post in a series to do with hydrogen in a Net Zero economy. The post Manufacture and Use of Hydrogen provided an overview to do with the production and use of hydrogen. Colors of Hydrogen described the color terms that are used to describe the different ways in which hydrogen is manufactured.
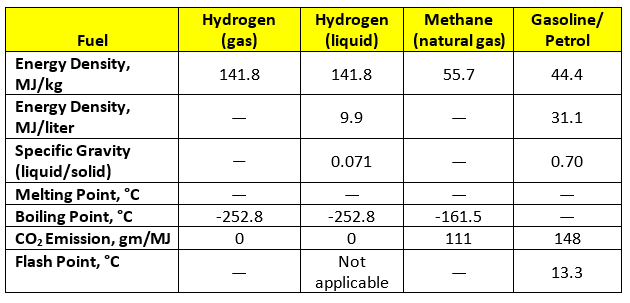
The following Table summarizes the key energy properties of hydrogen, both as a gas and as a liquid. Also provided is equivalent information for methane (natural gas) and gasoline (petrol).
The following insights can be drawn from the Table.
-
On a weight basis hydrogen has a high energy density — around three times greater than most fossil fuels. This high energy density makes hydrogen an attractive replacement for the fossil fuels, such as methane and gasoline.
-
Hydrogen has a very, very low boiling point. -253°C is just 20 degrees above absolute zero. Hence it requires a lot of energy to convert the gas to a liquid. Liquefied hydrogen is currently used in special applications, such as space missions. In such cases issues to do with thermodynamic efficiency are not critical. However, they become a serious concern when hydrogen is being considered as a mass market fuel.
-
The specific gravity of the liquid is low. Hence its energy density on a volume basis is only a third of the value for gasoline and other liquid fuels. In round numbers, a tank of liquid hydrogen delivers only a third of the energy of a tank of gasoline. This low specific gravity negates the effect of having high energy density on a weight basis.
-
Hydrogen has wide flammability limits and low minimum ignition energy. These properties create safety concerns, an issue that we will discuss in future posts.
-
The fact that hydrogen does not emit CO2 does not mean that it has no greenhouse impact. When burned in air the gas creates nitrogen oxides, some of which are potent greenhouse gases.